Mrna Influenza Vaccine
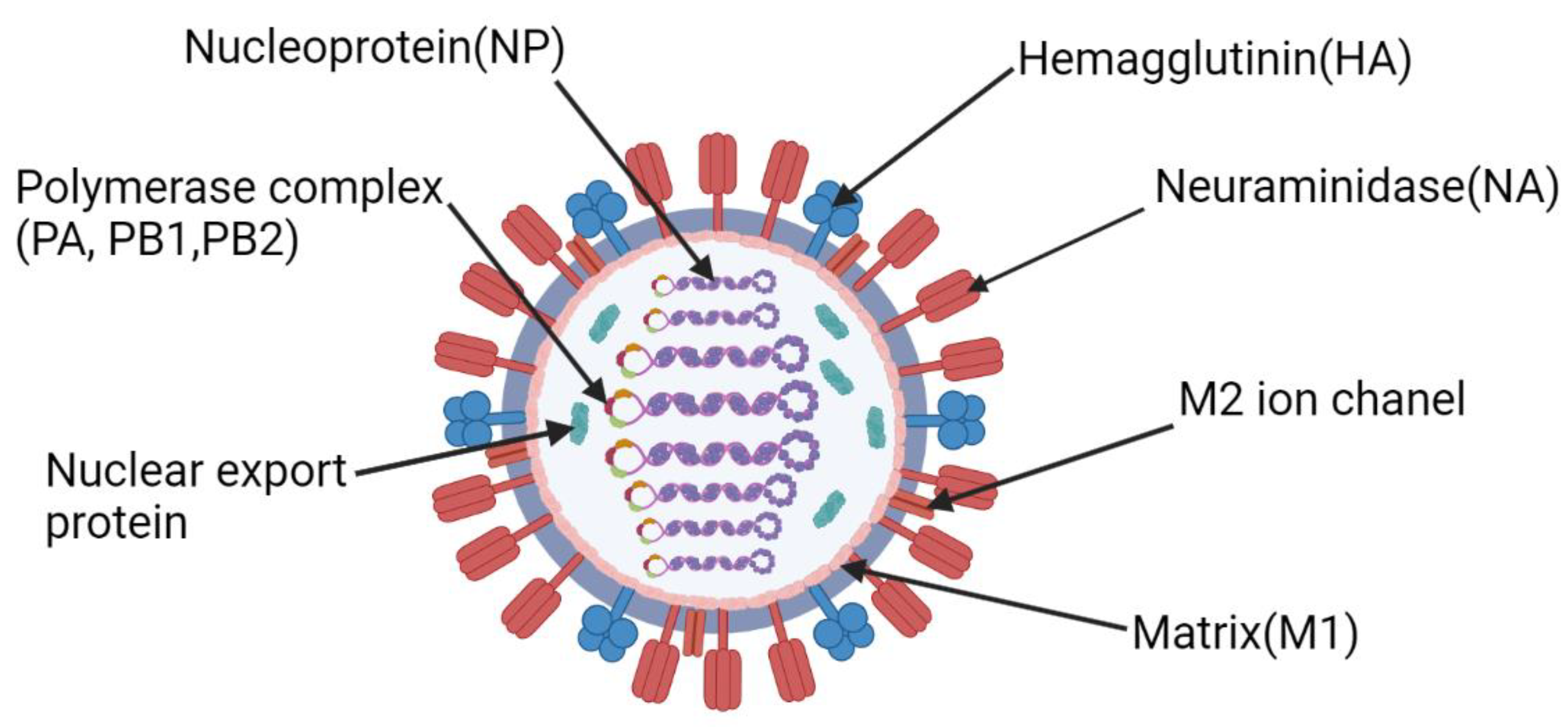
Vaccines Free Full Text Universal Flu Mrna Vaccine Promises Prospects And Problems A recent study showed that a seasonal quadrivalent influenza mrna 1010 vaccine encoding four haemagglutinin proteins (h1n1, h3n2, b victoria, and b yamagata) was safe for healthy adults over 18 years of age and induced superior haemagglutinin inhibition antibody titres to influenza a components compared with a standard inactivated seasonal. First phase 3 efficacy study to be conducted using an mrna based influenza vaccine; study will enroll 25,000 u.s. adults 18 years and older influenza causes 140,000 to 710,000 hospitalizations and 12,000 to 52,000 deaths in the u.s. every year1 mrna based vaccines require only the genetic sequences of the viruses, enabling more flexible, rapid manufacturing which may lead to improved strain.
New Mrna Vaccine To Fight 20 Known Subtypes Of Influenza Penn Today Unlike the vrc’s earlier vaccine, the h1ssf 3928 mrna lnp vaccine candidate uses a messenger rna (mrna) platform. by developing and testing a variety of different platforms for a universal flu vaccine, researchers are more likely to find one that is both safe and provides strong and broad immunity against a variety of strains. The combination candidate consists of pfizer’s mrna based influenza vaccine candidate with the companies’ licensed covid 19 vaccine. the phase 3 trial measured two primary immunogenicity objectives (immunogenicity against sars cov 2 as well as immunogenicity against influenza a and b), of which one was met. Testing the mrna flu vaccine candidate. in july, 2022, pfizer reported positive clinical results in its phase 2 trial of the mrna flu vaccine candidate. “the data from that trial suggest a strong t cell immune response in addition to good safety and tolerability in trial participants," says welch. t cells are white blood cells that recognize. Quadrivalent seasonal hemagglutinin mrna vaccination protects against a california 04 2009 infection following a single dose. as seasonal influenza vaccines are administered annually as a single.
An Mrna Universal Vaccine For Influenza Testing the mrna flu vaccine candidate. in july, 2022, pfizer reported positive clinical results in its phase 2 trial of the mrna flu vaccine candidate. “the data from that trial suggest a strong t cell immune response in addition to good safety and tolerability in trial participants," says welch. t cells are white blood cells that recognize. Quadrivalent seasonal hemagglutinin mrna vaccination protects against a california 04 2009 infection following a single dose. as seasonal influenza vaccines are administered annually as a single. In a recent study, a 20 valent mrna vaccine elicited immunity against influenza virus in mice. these results, combined with the success of mrna vaccines against covid 19, augur well for a new type. A vaccine using mrna technology induced an immune response in mice and ferrets against 20 different types of influenza. it also provided the animals protection against death from flu strains not included in the vaccine, showing its potential to help prevent future flu pandemics. influenza virus particles isolated from a patient sample and.
How Mrna Vaccines From Pfizer And Moderna Work Why They Re A Breakthrough And Why They Need To In a recent study, a 20 valent mrna vaccine elicited immunity against influenza virus in mice. these results, combined with the success of mrna vaccines against covid 19, augur well for a new type. A vaccine using mrna technology induced an immune response in mice and ferrets against 20 different types of influenza. it also provided the animals protection against death from flu strains not included in the vaccine, showing its potential to help prevent future flu pandemics. influenza virus particles isolated from a patient sample and.

First People Dosed With A Quadrivalent Seasonal Flu Mrna Vaccine

Comments are closed.